Abstract
Background: Pegaspargase (PEG) is integral in the treatment of patients with acute lymphoblastic leukemia/lymphoma (ALL/LBL). However, full exposure to all doses of PEG can be limited by hypersensitivity reactions (HSR), which necessitate a switch to alternative asparaginase formulations. Previous studies in the pediatric population evaluating the use of premedication with antihistamines (H1 and H2) to decrease the incidence of HSR have largely been retrospective and showed mixed results. We conducted the first prospective, multicenter study to evaluate the efficacy of premedicating pediatric and adolescent patients receiving PEG with H1 and H2 blockers to decrease the rate of HSR. We report on our interim analysis with a special focus on ethnic disparities in asparaginase activity and HSR.
Methods: We enrolled patients with ALL/LBL treated according to a Children's Oncology Group protocol at six centers across the US. Starting with the first dose of PEG in consolidation and subsequently, patients received an H1 blocker (diphenhydramine) and an H2 blocker (ranitidine, famotidine or cimetidine) 30-60 minutes prior to every dose of PEG, which was infused over 60-120 minutes. TDM was performed at 7-10 and 14-17 days post each PEG infusion. Therapeutic asparaginase activity was defined as ≥ 0.1 IU/mL (≥100 IU/L ) at 7-10 days post infusion and greater than the lower level of quantification at 14-17 days. We observed for silent inactivation (subtherapeutic levels without symptoms) and accelerated clearance (levels below quantification at 14-17 days without symptoms). Ethnicity differences were assessed using Chi-square and independent t-tests, increased odds of HSR by GLM procedure, and average asparaginase activity across treatments using GEE analyses.
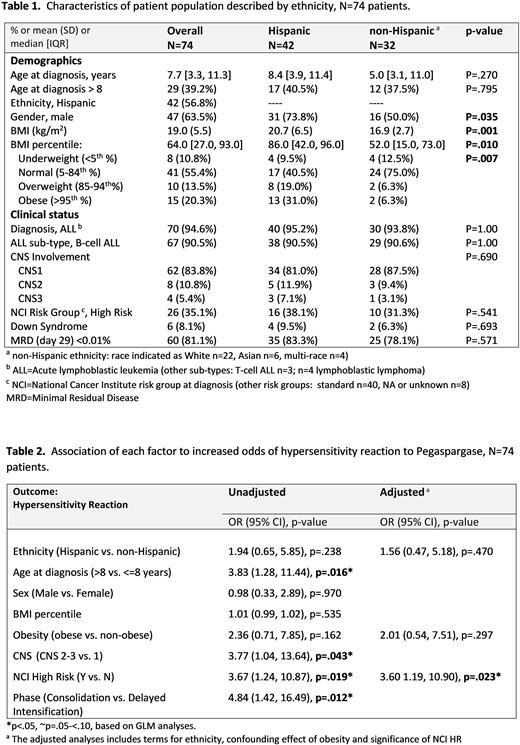
Results: We report on the 74 patients enrolled from 10/2019 to 7/2021 who completed all PEG therapy at the time of analysis. The median age at diagnosis was 7.7 years [range 1.3-19.8], 63.5% were male, 90.5% had B-ALL, 35.1% were NCI High Risk (HR) at diagnosis, and 8.1% had Down Syndrome (Table 1). A majority self-identified as Hispanic ethnicity (56.8%). We noted a disparity in obesity/overweight status by ethnicity. Overall, 50.0% of Hispanics were obese (31.0%) or overweight (19.0%) compared to 12.6% of non-Hispanics (6.3% obese and 6.3% overweight), p=.007. The average body mass index (BMI, kg/m2) in Hispanic compared to non-Hispanic patients was 20.7 (SD=6.5) vs. 16.9 (SD=2.7), p=.001.
A total of 157 infusions of PEG was administered to 74 patients. The average dose of PEG was 2780 IU with an average time of infusion of 128 minutes in those without HSR. The incidence of HSR for Hispanic patients was 31% compared to 18.8% for non-Hispanics, although this was non-significant (p=0.234). In 19 patients who experienced HSR, 84% were Grade 3 and one was a Grade 4. Most HSR occurred during consolidation (79.9%). Silent inactivation occurred in 3 patients (4%) and one patient experienced delayed clearance. The average asparaginase activity level at 7-10 and 14-17 days was 1.03 IU/mL (SD=0.35) and 0.71 IU/mL (SD=0.30), respectively. There was no significance in asparaginase activity levels between Hispanics and non-Hispanics (p>.05). Overall, 81% of all patients achieved a (-) minimal residual disease (MRD) at the end of induction with no difference in Hispanic (83.3%) versus non-Hispanics (78.1%). In an adjusted analysis to evaluate potential determinants of HSR, we noted that NCI HR at diagnosis (p=0.019) was associated with increased incidence of HSR, while obesity, sex and ethnicity were not (Table 2). Interestingly, age >8 years was associated with higher odds of HSR unadjusted for NCI HR (OR=3.83, p=.016).
Conclusion: We found that the two factors which increase the odds of HSR are age > 8 years and NCI high risk at diagnosis. We will be able to confirm the efficacy of premedication with a larger cohort upon the completion of all patients enrolled. Both silent inactivation and delayed clearance was infrequent. Although the standard therapeutic asparaginase activity is widely accepted to be ≥ 0.1 IU/mL, we observed supratherapeutic levels at 1 and 2 weeks post PEG at 1.03 IU/mL and 0.71, respectively. We are currently performing additional analysis to evaluate PEG related toxicity in various ethnic groups, determining the single nucleotide polymorphisms (SNPs) involved in HSR and toxicity, and creating a dose model based on pharmacokinetic analysis.
Disclosures
Huynh:Servier Pharmaceuticals: Research Funding. Agrawal:YmAbs Therapeutics: Membership on an entity's Board of Directors or advisory committees. Phillips:Incyte: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. August:Beam Therapeutics: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Guest:Jazz Pharmaceuticals: Speakers Bureau; Syndax Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.